Trastuzumab Botidotin: Ushering HER2-Targeted Therapy into a New Era of Dual-Epitope Synergy
As precision oncology continues to advance, HER2 (human epidermal growth factor receptor 2) has become one of the most mature and clinically valuable therapeutic targets across multiple solid tumors. With an improved understanding of tumor heterogeneity and mechanisms of drug resistance, the limitations of single-target, single-mechanism treatment strategies have become increasingly evident. Enhancing therapeutic efficacy while delaying or overcoming resistance has therefore become a central focus in the ongoing evolution of HER2-targeted therapies.
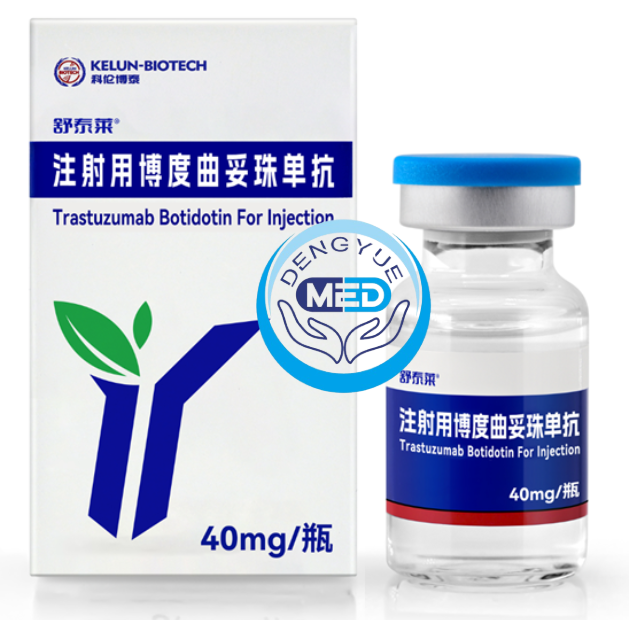
Trastuzumab botidotin was developed in response to this unmet need. As a next-generation HER2-targeted antibody, it introduces an innovative dual-epitope synergistic design, offering new approaches and possibilities for the treatment of HER2-positive malignancies.
Innovative Mechanism: Dual Binding to Strengthen HER2 Signal Inhibition
Trastuzumab botidotin is a bispecific antibody targeting HER2, capable of simultaneously binding to two distinct epitopes on the HER2 receptor. Unlike conventional monoclonal antibodies that interact with a single binding site, this dual-epitope design provides multiple advantages:
-
More comprehensive blockade of HER2 signaling pathways
-
Enhanced receptor internalization and degradation, reducing HER2 expression on the cell surface
-
More effective suppression of tumor cell proliferation signals
-
Enhanced antibody-dependent cellular cytotoxicity (ADCC)
This synergistic mechanism enables more stable and durable HER2 inhibition within the complex tumor microenvironment and offers a novel strategy for patients who show limited responses to prior HER2 monoclonal antibody therapies.
Clinical Development: Demonstrating Potential Across Multiple HER2-Positive Tumors
Current clinical studies indicate that Trastuzumab botidotin demonstrates encouraging antitumor activity across a range of HER2-expressing or HER2-amplified cancers, particularly in the following indications:
-
HER2-positive gastric cancer and gastroesophageal junction cancer
-
HER2-positive breast cancer
-
Other HER2-driven solid tumors
Importantly, clinically meaningful efficacy signals have been observed in some patients previously treated with HER2-targeted therapies, suggesting potential value in post-resistance or later-line treatment settings. Ongoing studies continue to explore its role across different lines of therapy and in various combination regimens.
Safety Profile: Predictable and Manageable Adverse Events
Based on available clinical data, Trastuzumab botidotin demonstrates a well-defined and predictable safety profile. The types of adverse events reported are generally consistent with those associated with HER2-targeted antibodies, primarily including gastrointestinal symptoms and infusion-related reactions.
Most adverse events are mild to moderate in severity and manageable with standard monitoring and supportive care, providing a foundation for further optimization in long-term treatment and combination therapy strategies.
Expanding the HER2 Treatment Paradigm: From Single-Site Inhibition to Precision Synergy
The development and clinical advancement of Trastuzumab botidotin reflect an important shift in HER2-targeted therapy—from early single-antibody, single-site blockade toward multi-epitope, multi-mechanism synergistic inhibition.
This approach not only enhances antitumor efficacy but also offers new tools to address tumor heterogeneity and delay the emergence of resistance, contributing to the continued refinement of HER2-targeted treatment paradigms.
Conclusion: Expanding Possibilities for Patients with HER2-Positive Tumors
As precision medicine continues to evolve, HER2-targeted therapy has progressed from addressing availability to optimizing treatment strategies. Through its innovative dual-epitope design, Trastuzumab botidotin introduces a new technical pathway and clinical vision for the management of HER2-positive cancers.
With the accumulation of further clinical trial data and real-world experience, Trastuzumab botidotin is expected to establish a clearer and more meaningful position within the HER2 treatment landscape, offering patients longer survival and improved therapeutic options. Meanwhile, global pharmaceutical distributor DengyueMed continues to bring more innovative medicines from China to the international market.