How Europe Is Transforming Drug Development with In-Silico Trials and Advanced Simulation Technologies
Introduction to the In-Silico Trials Market
The in-silico trials market is emerging as a transformative segment within the healthcare and life sciences industry. Computational modelling and simulation technologies are reshaping how medical products are designed, tested, and validated before entering traditional clinical trials. These digital trials rely on advanced algorithms, artificial intelligence, and virtual patient models to predict safety, efficacy, and performance. As healthcare systems seek faster innovation cycles and cost efficient solutions, the adoption of simulation driven research continues to accelerate across pharmaceutical, biotechnology, and medical technology sectors.
Market Size and Growth Trajectory
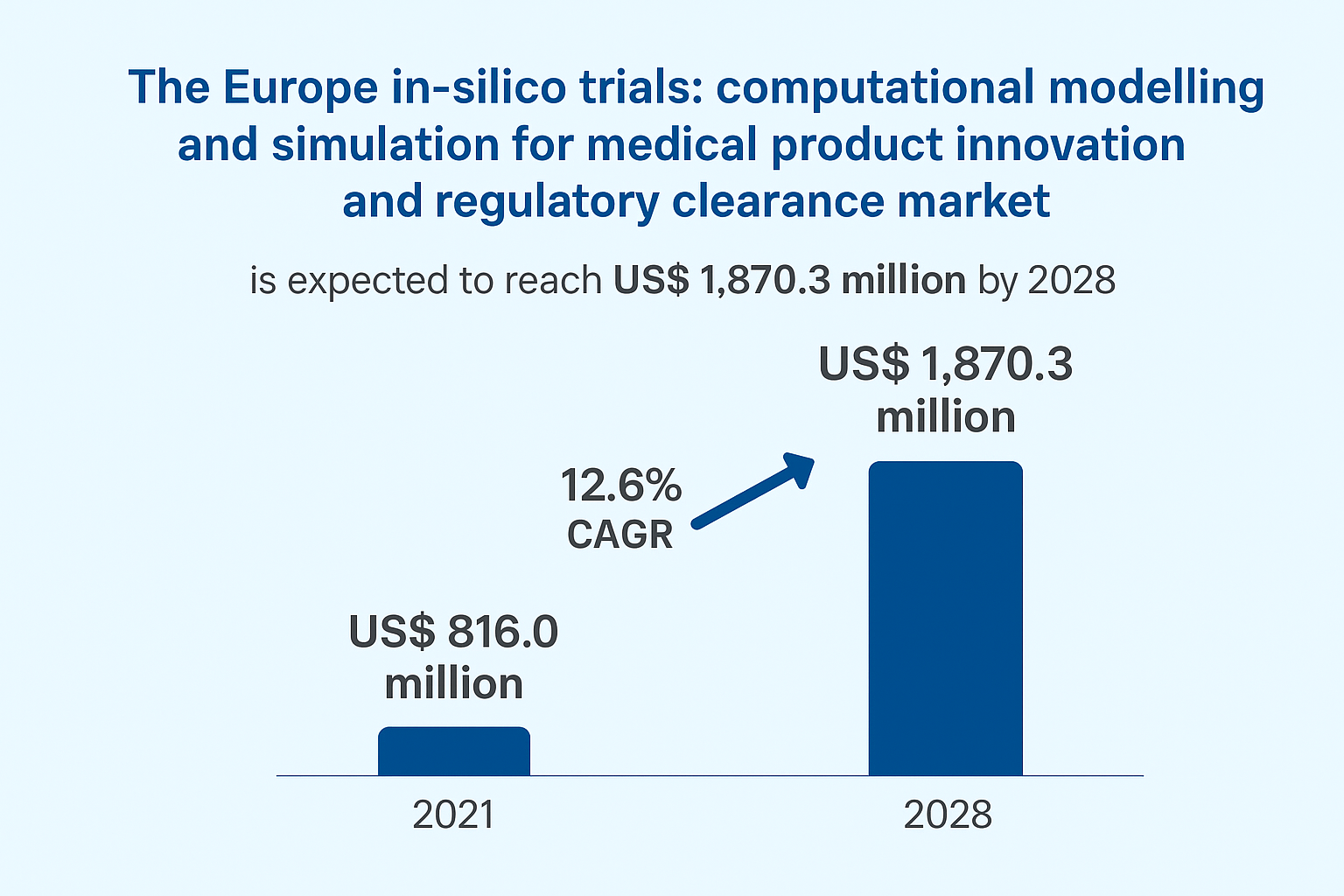
The market is projected to grow from US$ 816.0 million in 2021 to US$ 1,870.3 million by 2028, reflecting a compound annual growth rate CAGR of 12.6% during the forecast period. This rapid growth highlights the increasing reliance on computational approaches to support drug discovery, medical device development, and regulatory decision making. Growing investments in digital health infrastructure, rising research costs, and the need for faster product approvals are contributing to strong market expansion.
Request Sample PDF Here:https://www.theinsightpartners.com/buy/TIPRE00029238
Cost and Time Advantages Over Traditional Trials
Traditional clinical trials are expensive, time consuming, and often limited in scope. In-silico approaches provide a powerful alternative by enabling researchers to test multiple hypotheses simultaneously with relatively modest increases in resources once the computational infrastructure is established. Virtual trials can generate statistically significant insights more quickly, reducing development timelines and minimizing financial risk. These benefits are encouraging organizations to integrate simulation technologies into their research pipelines.
Growing Acceptance in Regulatory Processes
Regulatory acceptance is becoming a major driver of market growth. Simulation based evidence is increasingly recognized as valuable support for regulatory submissions and evaluations. Digital trials can complement traditional evidence by providing additional insights into safety and performance. As regulatory bodies continue to acknowledge the reliability of computational data, the adoption of virtual trials is expected to grow steadily across healthcare industries.
Market Segmentation by Organization Size and Offerings
The market is segmented by organization size into small and medium enterprises and large organizations. Large enterprises often lead adoption due to their substantial research budgets and technological resources. However, smaller companies are rapidly embracing simulation tools to enhance innovation and remain competitive. By offerings, the market includes products, platforms, and services. Software platforms and simulation services are gaining significant traction as organizations seek scalable and flexible solutions.
Application Areas Driving Market Demand
Applications of in-silico trials span multiple stages of product development. Key application areas include product design and discovery, product development, pre clinical targeting, and assessment of drugs and biomedical products. These applications allow companies to evaluate performance, optimize design, and predict outcomes before moving to physical testing. The ability to test multiple design scenarios virtually is accelerating innovation across healthcare sectors.
Clinical Indications Expanding Use Cases
Simulation technologies are used across a wide range of clinical indications. These include cardiovascular diseases, neurodegenerative disorders, oncology, rare diseases, metabolic conditions, immune based diseases, and infectious diseases. The ability to model complex disease mechanisms and simulate patient responses is expanding the scope of virtual trials and increasing their value in medical research.
Regional Market Landscape
Key markets include Germany, the United Kingdom, France, Spain, Italy, and other countries across the region. Strong research infrastructure, supportive regulatory environments, and high investment in healthcare innovation are driving adoption across these markets.
Key Players in the Market
Leading companies are actively investing in advanced modelling technologies and strategic partnerships to strengthen their market presence.
-
InSilicoTrials Technologies
-
Feops
-
CADFEM Medical GmbH
-
Dassault Systèmes SE
-
Virtonomy GmbH
-
Certara Inc.
-
Computational Life
-
NOVA
-
TwInsight Medical
-
Ansys Inc.
Future Outlook
The future of the in-silico trials market is highly promising as healthcare organizations continue to embrace digital transformation. Advances in artificial intelligence, machine learning, and high performance computing will further enhance the accuracy and reliability of simulation models. Increasing collaboration between technology providers, pharmaceutical companies, and regulatory agencies will accelerate adoption and standardization.






