iso 13485 certification03
ISO 13485 Certification: Ensuring Quality in Medical Device Manufacturing
Introduction to ISO 13485
ISO 13485 is an internationally recognized standard that specifies requirements for a quality management system in the medical device industry. Organizations involved in the design, production, installation, and servicing of medical devices rely on this certification to demonstrate their commitment to product safety, regulatory compliance, and consistent quality. ISO 13485 helps manufacturers meet customer expectations and comply with global regulatory requirements.
Importance of ISO 13485 Certification
Achieving ISO 13485 certification is critical for organizations seeking to operate in global medical device markets. It ensures that manufacturers produce safe and reliable devices while maintaining a robust quality management system. The certification also builds trust with regulatory bodies, customers, and business partners. By focusing on risk management and process control, ISO 13485 helps reduce product failures, recalls, and non-compliance issues.
Key Requirements of the Standard
ISO 13485 emphasizes stringent documentation, risk-based decision-making, and regulatory compliance. It requires organizations to establish controlled processes for product design, development, and production. The standard also mandates clear procedures for traceability, internal audits, corrective actions, and training. A strong emphasis is placed on product lifecycle management to ensure safety from concept to disposal.
Benefits for Organizations
ISO 13485 certification provides significant advantages, including access to international markets, improved operational efficiency, and stronger process control. It enhances brand credibility and customer confidence by demonstrating compliance with industry best practices. The certification also supports continuous improvement, helping companies reduce waste, improve product quality, and ensure regulatory readiness.
Certification Process Overview
The certification journey begins with a gap analysis to evaluate current systems against ISO 13485 requirements. This is followed by documentation preparation, system implementation, and internal audits. Once ready, an accredited certification body conducts a two-stage audit to assess compliance. Upon successful evaluation, the organization receives ISO 13485 certification and undergoes periodic surveillance audits to maintain it.
Role in Regulatory Compliance
ISO 13485 supports compliance with global regulations such as EU MDR and U.S. FDA requirements. It acts as a foundation for demonstrating conformity in international markets. By aligning quality management systems with regulatory expectations, organizations can streamline approvals, reduce compliance risks, and accelerate time-to-market for medical devices.
Conclusion
ISO 13485 certification is a vital step for medical device manufacturers committed to safety, quality, and regulatory excellence. By implementing this standard, organizations enhance their competitive edge and strengthen their quality culture, ultimately contributing to better patient outcomes and global healthcare advancement.
Nach Verein filtern
Read More
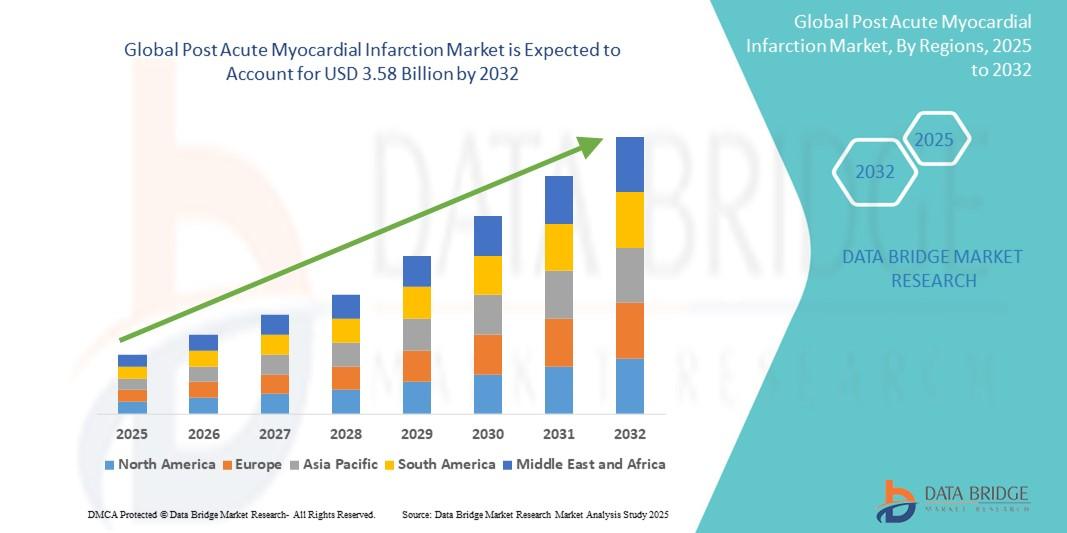
"Comprehensive Outlook on Executive Summary Post Acute Myocardial Infarction Market Size and Share CAGR Value The global post acute myocardial infarction market size was valued at USD 2.18 billion in 2024 and is expected to reach USD 3.58 billion by 2032, at a CAGR of 6.40% during the forecast period Post Acute Myocardial Infarction Market research...
Introduction: Building Your Dream Squad in FC 26 Ultimate Team Building your ideal FC 26 Ultimate Team is a highly personal journey, as each manager’s style and preferences are unique. Everyone approaches their squad differently, prioritizing different qualities in players and team structure. Selecting top players involves more than just focusing on high ratings—consider how well a...

Cricket is followed with unmatched passion across India, and online betting has become a popular way for fans to enjoy matches with added excitement. As the demand for reliable betting platforms grows, Mahakal Book has established itself as a trusted and well-known name among online cricket betting users. What Is Mahakal Book? Mahakal Book is an online betting platform that allows users to...

Executive Summary Automotive Glove Box Market : Global automotive glove box market was valued at USD 152.5 million in 2021 and is expected to reach USD 196.20 million by 2029, registering a CAGR of 3.20% during the forecast period of 2022-2029 A large-scale Automotive Glove Box Market business report endows with a profound overview of product specification, product type, production...

Netflix Unveils 'Castlevania' Series: A New Chapter in Vampire Entertainment Netflix has announced an exciting addition to its original programming lineup with the upcoming release of 'Castlevania,' an animated series based on the popular video game franchise. Set to premiere later in 2017, the show will follow the journey of the disgraced Belmont family's last descendant as he battles Dracula...


