iso 13485 certification03
ISO 13485 Certification: Ensuring Quality in Medical Device Manufacturing
Introduction to ISO 13485
ISO 13485 is an internationally recognized standard that specifies requirements for a quality management system in the medical device industry. Organizations involved in the design, production, installation, and servicing of medical devices rely on this certification to demonstrate their commitment to product safety, regulatory compliance, and consistent quality. ISO 13485 helps manufacturers meet customer expectations and comply with global regulatory requirements.
Importance of ISO 13485 Certification
Achieving ISO 13485 certification is critical for organizations seeking to operate in global medical device markets. It ensures that manufacturers produce safe and reliable devices while maintaining a robust quality management system. The certification also builds trust with regulatory bodies, customers, and business partners. By focusing on risk management and process control, ISO 13485 helps reduce product failures, recalls, and non-compliance issues.
Key Requirements of the Standard
ISO 13485 emphasizes stringent documentation, risk-based decision-making, and regulatory compliance. It requires organizations to establish controlled processes for product design, development, and production. The standard also mandates clear procedures for traceability, internal audits, corrective actions, and training. A strong emphasis is placed on product lifecycle management to ensure safety from concept to disposal.
Benefits for Organizations
ISO 13485 certification provides significant advantages, including access to international markets, improved operational efficiency, and stronger process control. It enhances brand credibility and customer confidence by demonstrating compliance with industry best practices. The certification also supports continuous improvement, helping companies reduce waste, improve product quality, and ensure regulatory readiness.
Certification Process Overview
The certification journey begins with a gap analysis to evaluate current systems against ISO 13485 requirements. This is followed by documentation preparation, system implementation, and internal audits. Once ready, an accredited certification body conducts a two-stage audit to assess compliance. Upon successful evaluation, the organization receives ISO 13485 certification and undergoes periodic surveillance audits to maintain it.
Role in Regulatory Compliance
ISO 13485 supports compliance with global regulations such as EU MDR and U.S. FDA requirements. It acts as a foundation for demonstrating conformity in international markets. By aligning quality management systems with regulatory expectations, organizations can streamline approvals, reduce compliance risks, and accelerate time-to-market for medical devices.
Conclusion
ISO 13485 certification is a vital step for medical device manufacturers committed to safety, quality, and regulatory excellence. By implementing this standard, organizations enhance their competitive edge and strengthen their quality culture, ultimately contributing to better patient outcomes and global healthcare advancement.
Categorii
Citeste mai mult
Beginning of a Creative Fashion Journey Comme des Garçons and Glo Gang have come together to bring a special side of streetwear. Both brands have their own identity, but when they meet, they build a new sense of fashion. This mix connects luxury fashion with modern street culture. The designs show freedom, self-expression, and confidence. People who wear these collections feel a strong...

Comprehensive Outlook on Executive Summary Cardiopulmonary Exercise Testing Market Size and Share The global cardiopulmonary exercise testing market size was valued at USD 3.82 billion in 2024 and is expected to reach USD 5.26 billion by 2032, at a CAGR of 4.10% during the forecast period. A competitive era calls for businesses to be equipped with...

"Executive Summary Garage and Service Station Market Value, Size, Share and Projections The garage and service station market size is valued at USD 3,639.15 million by 2028 and is expected to grow at a compound annual growth rate of 10.2% over the forecast period of 2021 to 2028. The top notch Garage and Service Station Market report explains current and future market trends...
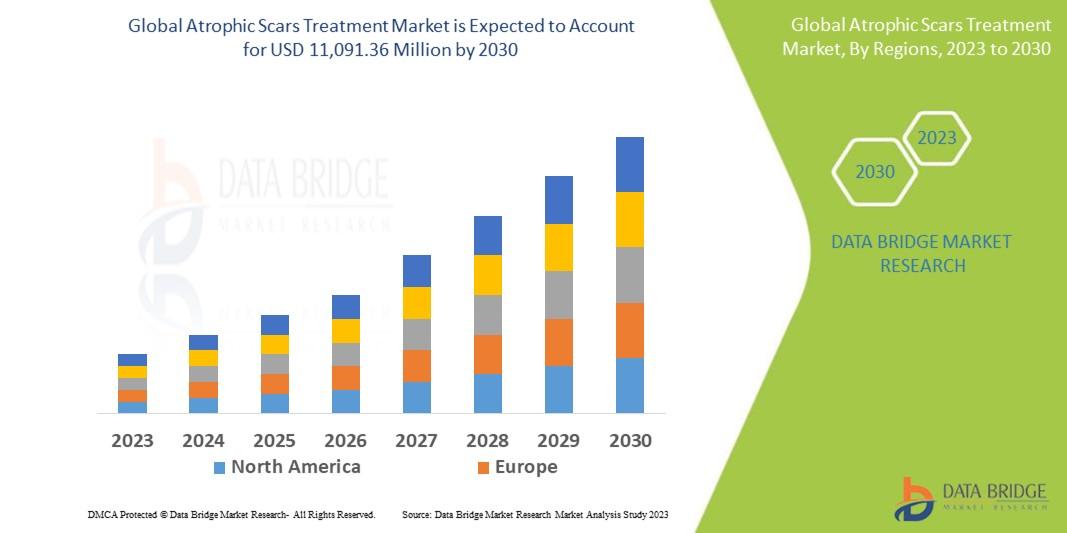
"Comprehensive Outlook on Executive Summary Retinal Detachment Treatment Market Size and Share Retinal detachment treatment market is expected to gain market growth in the forecast period of 2022-2029. Data Bridge Market Research analyses the market to account to grow at a CAGR of 6.5% in the above mentioned forecast period. Retinal Detachment Treatment Market research report is...

Download verified BCBA exam dumps from HelloDumps. Enjoy accurate questions, multiple practice formats, instant download, and 25% OFF. Boost your chances of passing on the first attempt. Best BACB BCBA Exam Dumps – Get 25% OFF at HelloDumps Looking for the most reliable BACB BCBA exam dumps? HelloDumps offers updated and verified BCBA exam questions that align with the latest BACB...


