iso 13485 certification03
ISO 13485 Certification: Ensuring Quality in Medical Device Manufacturing
Introduction to ISO 13485
ISO 13485 is an internationally recognized standard that specifies requirements for a quality management system in the medical device industry. Organizations involved in the design, production, installation, and servicing of medical devices rely on this certification to demonstrate their commitment to product safety, regulatory compliance, and consistent quality. ISO 13485 helps manufacturers meet customer expectations and comply with global regulatory requirements.
Importance of ISO 13485 Certification
Achieving ISO 13485 certification is critical for organizations seeking to operate in global medical device markets. It ensures that manufacturers produce safe and reliable devices while maintaining a robust quality management system. The certification also builds trust with regulatory bodies, customers, and business partners. By focusing on risk management and process control, ISO 13485 helps reduce product failures, recalls, and non-compliance issues.
Key Requirements of the Standard
ISO 13485 emphasizes stringent documentation, risk-based decision-making, and regulatory compliance. It requires organizations to establish controlled processes for product design, development, and production. The standard also mandates clear procedures for traceability, internal audits, corrective actions, and training. A strong emphasis is placed on product lifecycle management to ensure safety from concept to disposal.
Benefits for Organizations
ISO 13485 certification provides significant advantages, including access to international markets, improved operational efficiency, and stronger process control. It enhances brand credibility and customer confidence by demonstrating compliance with industry best practices. The certification also supports continuous improvement, helping companies reduce waste, improve product quality, and ensure regulatory readiness.
Certification Process Overview
The certification journey begins with a gap analysis to evaluate current systems against ISO 13485 requirements. This is followed by documentation preparation, system implementation, and internal audits. Once ready, an accredited certification body conducts a two-stage audit to assess compliance. Upon successful evaluation, the organization receives ISO 13485 certification and undergoes periodic surveillance audits to maintain it.
Role in Regulatory Compliance
ISO 13485 supports compliance with global regulations such as EU MDR and U.S. FDA requirements. It acts as a foundation for demonstrating conformity in international markets. By aligning quality management systems with regulatory expectations, organizations can streamline approvals, reduce compliance risks, and accelerate time-to-market for medical devices.
Conclusion
ISO 13485 certification is a vital step for medical device manufacturers committed to safety, quality, and regulatory excellence. By implementing this standard, organizations enhance their competitive edge and strengthen their quality culture, ultimately contributing to better patient outcomes and global healthcare advancement.
Categorieën
Read More
Global Demand Outlook for Executive Summary Foodservice Disposables Market Size and Share The global foodservice disposables market was valued at USD 91.81 billion in 2024 and is expected to reach USD 201.15 billion by 2032. During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 10.30%, primarily driven by the growing demand for...

Benefits of Using Certification Exam Preparation Material Certification exams help people grow in their careers. They help you get better jobs. They also help you earn more money. Many students want to pass the 19 exam on the first attempt. This exam is offered by PeopleCert. However, exam preparation can feel difficult. There are many books to read and many topics to cover. This is why using...

When it comes to finding your perfect scent, Elyon Dubai understands that testing a perfume before making a purchase is crucial. A fragrance can evoke memories, set moods, and even become a signature part of your identity. But with so many notes, concentrations, and personal preferences to consider, the process of testing perfumes can feel overwhelming. To help you make the right choice,...

Learn how to choose indexable tools based on performance, cost, and durability. A complete buying guide for efficient and cost-effective CNC machining. A Complete Buying Guide to Indexable Tools: Performance, Cost, and Durability Choosing the right cutting solution is critical for manufacturers aiming to achieve precision, productivity, and cost efficiency. In modern...

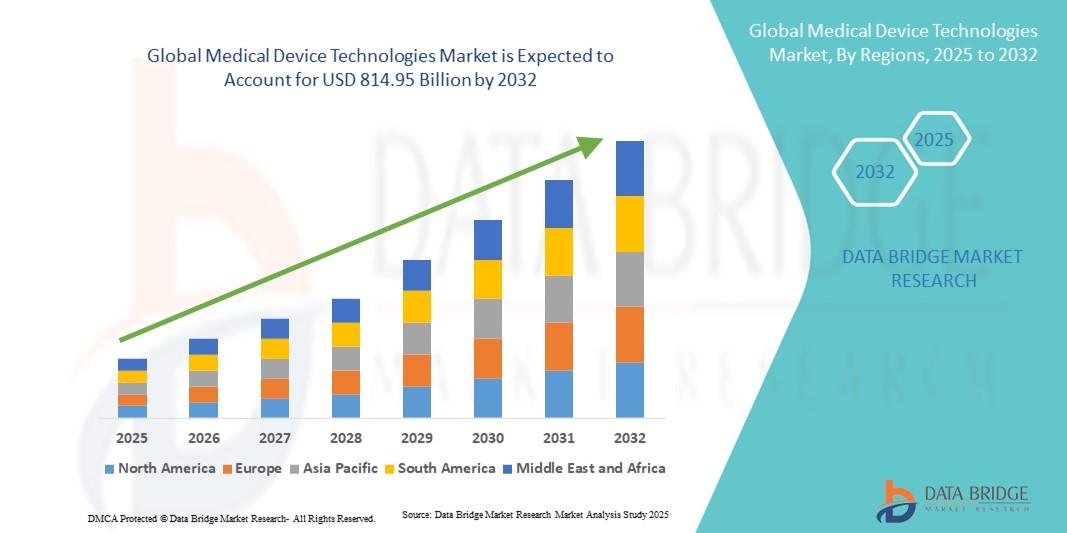
"Executive Summary Medical Device Technologies Market Size and Share Analysis Report CAGR Value The global medical device technologies market size was valued at USD 539.14 billion in 2024 and is expected to reach USD 814.95 billion by 2032, at a CAGR of 5.30% during the forecast period With the use of few steps or the combination of several steps, the...